ACLAR® Flex 380
overwraps, and lidding.
General Properties
Access the complete datasheet details for ACLAR® Flex 380 when you create your free account with Prospector. You’ll find complete information on physical, mechanical and hardness specs.
Technical Datasheet (English)
Download DocumentProcessing
Find specific processing information for ACLAR® Specialty Film as well as general information for the Polychlorotrifluoroethylene generic family. Register or Sign In for more information.
Where To Buy
You can purchase ACLAR® Flex 380 from 1 distributors or manufacturers. Register or Sign In for more information.
Find UL Yellow Cards
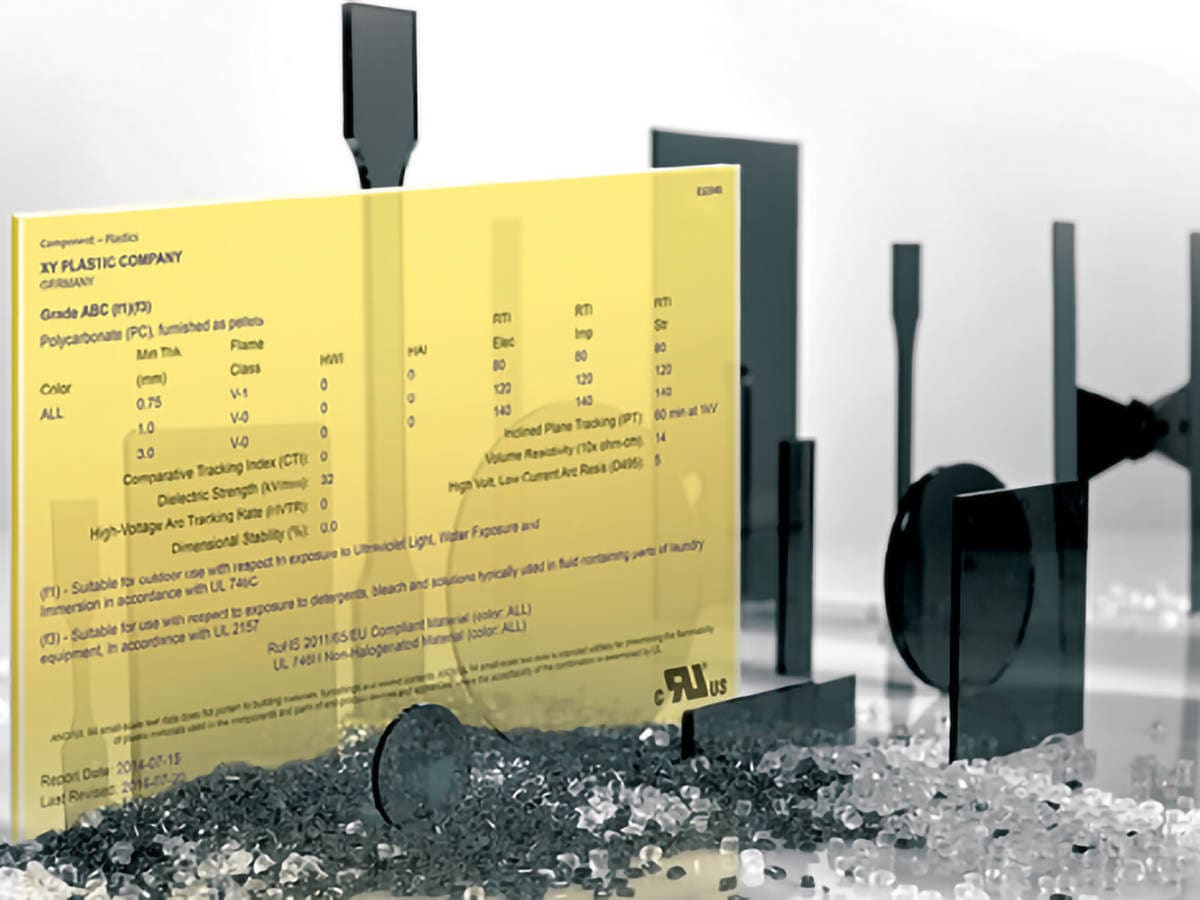
UL's Yellow Cards are a globally recognized safety and quality guarantee, a certified demonstration of how plastics have met a specific set of performance credentials.

PROSPECTOR
Find solutions, not just materials.
When you join Prospector, you leverage the dynamic tools and features to find solutions fast.
Register Today






