MedSelect™ P5M6K-080
Features: Clarified. Produced without animal derived components or phthalates
Sterilization: Gamma radiation, EtO, E-beam, and autoclave
Applications: Syringes, medical device components
General Properties
Access the complete datasheet details for MedSelect™ P5M6K-080 when you create your free account with Prospector. You’ll find complete information on physical, mechanical and hardness specs.
Technical Datasheet (English)
Download DocumentProcessing
Find specific processing information for MedSelect™ Polypropylene as well as general information for the Polypropylene Random Copolymer generic family. Register or Sign In for more information.
Where To Buy
You can purchase MedSelect™ P5M6K-080 from 7 distributors or manufacturers. Register or Sign In for more information.
Find UL Yellow Cards
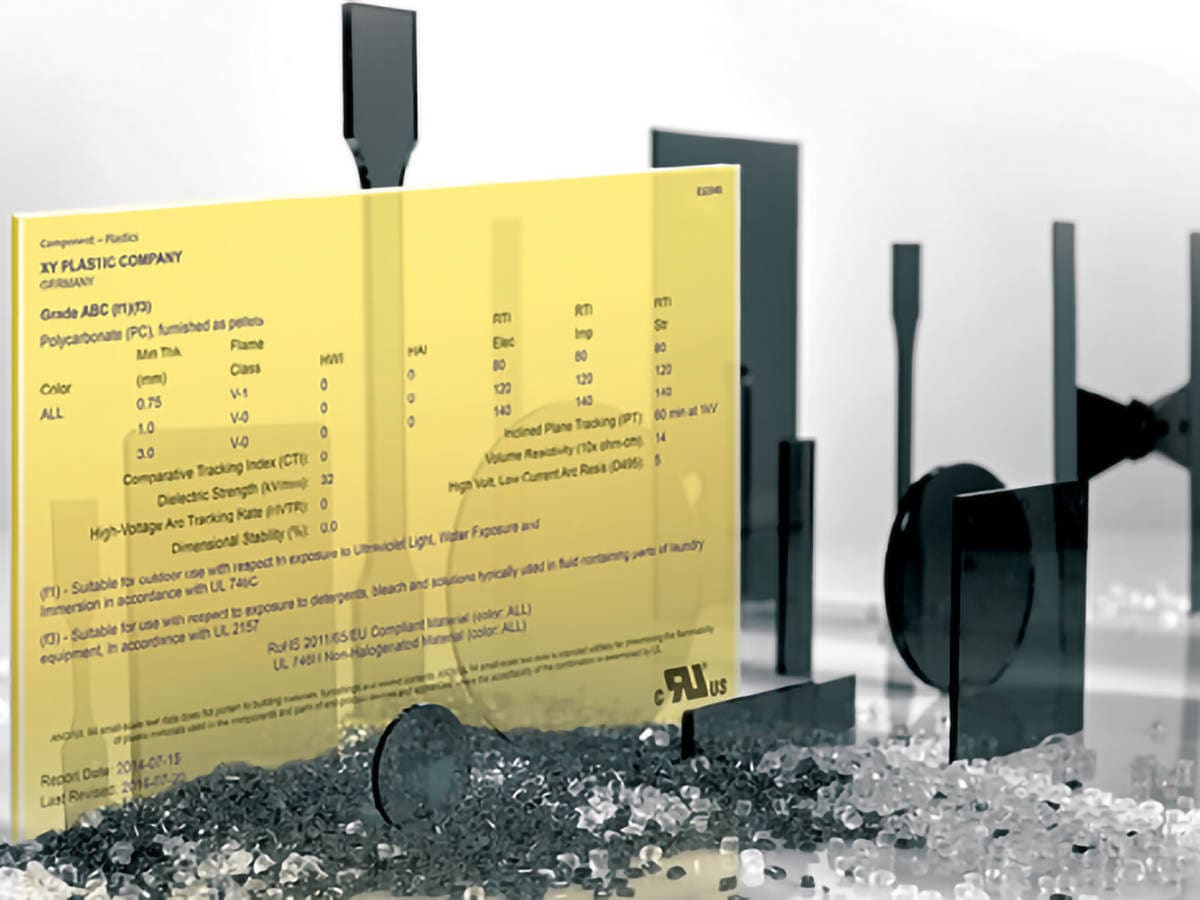
UL's Yellow Cards are a globally recognized safety and quality guarantee, a certified demonstration of how plastics have met a specific set of performance credentials.

PROSPECTOR
Find solutions, not just materials.
When you join Prospector, you leverage the dynamic tools and features to find solutions fast.
Register Today






